Thank you for the great response to our first newsletter of the term!
Going forward, we will be posting these at the start of each week. This will give you a sneak preview of what your kids will be learning in the days to come and provide you with time to prepare for an enthusiastic science chat with them on the drive home.
So here's what we have in store for your future scientists this week...
SCIENCE BEHIND THE WAND
If the fires of last week weren't enough to ignite excitement for the term ahead, then this week's spell - Confringo - will surely get us going with a bang! Building on last week's knowledge of the fire triangle, we will take great fuels, like rubbing alcohol and lycopodium, an energetic powder made from the spores of a moss, and thoroughly mix them with oxygen to create explosive fuel-air mixtures. Flames will rise, fires will whoosh out of flasks, and dragon breath will be unleashed. However, by trapping all these hot gases and building enough pressure, we will create mini-explosions that will send ping pong balls flying through the air.
MYTH BUSTERS
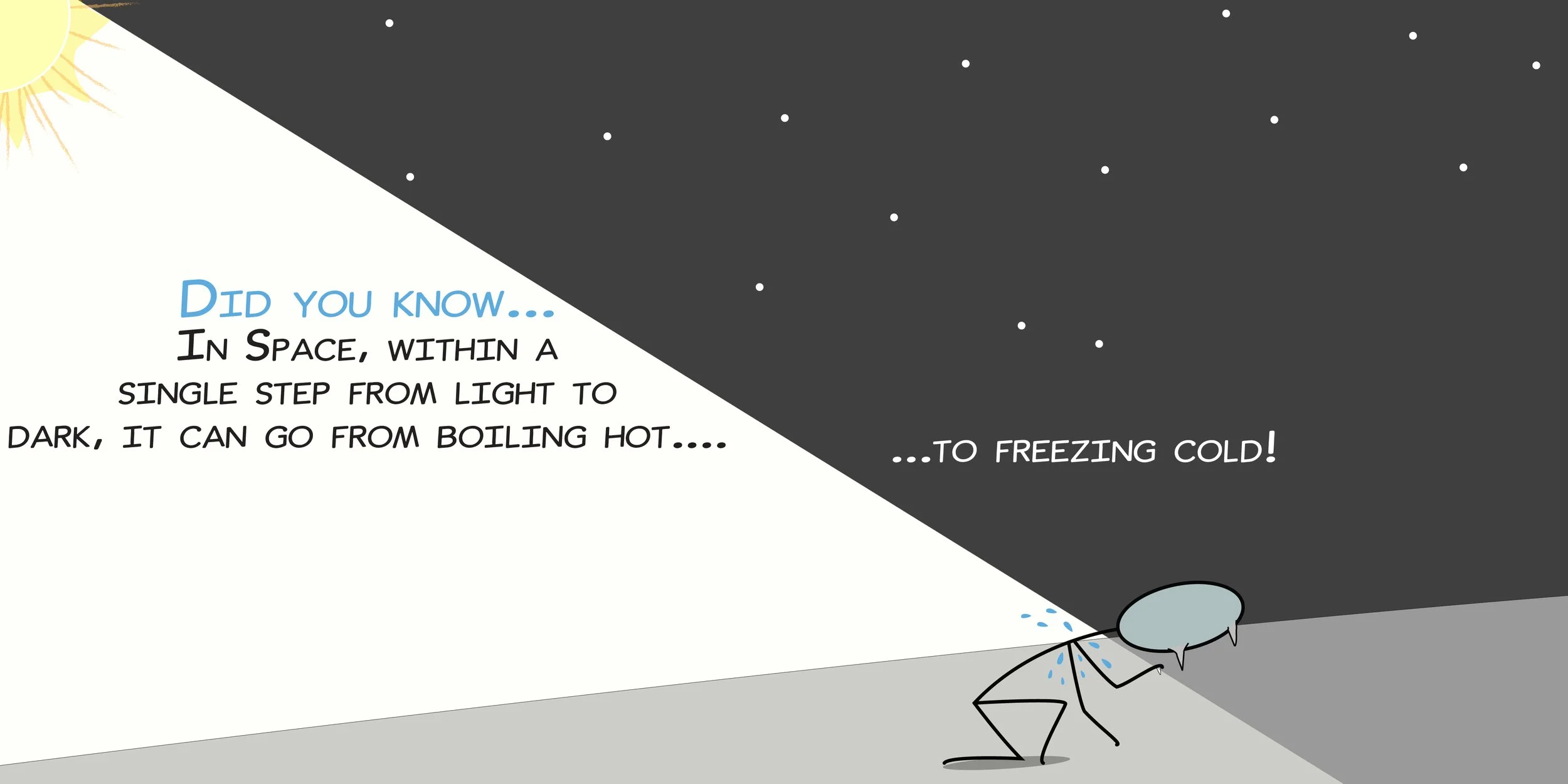
As we make our way back from the Sun, we will slow down this week to answer a few myths about Space. More specifically, myths about Space's supposed hostility to life. What actually happens to human bodies in the vacuum of Space? Would they explode? Could they shout for help? Before we answer these pressing questions, we will investigate the nature of sound and how it travels through solids, liquids and gases, as well as experiment with the air pressure within us and around us to gravity-defying results. Then, it's time to fire up the vacuum chamber to recreate a tiny bit of Space within the lab and sort fact from fiction.
PERIODIC PIONEERS
Hydrogen, being the simplest of the elements, makes up 90% of all the atoms in the Universe. It is so prevalent that much of chemistry is a dance where hydrogen atoms switch partners. We get hands-on with one such dance this week as we use the pH scale to admire hydrogen's moves and identify acids and bases. At the extremes of pH, these chemicals can be deadly, but moderate ones are found all around us. By squeezing the juice out of some red cabbage, we will extract an indicator that will use colour to sort household chemicals by their pH. Then, using colour as their guide again, can we find the right mix to neutralise these acids and bases?